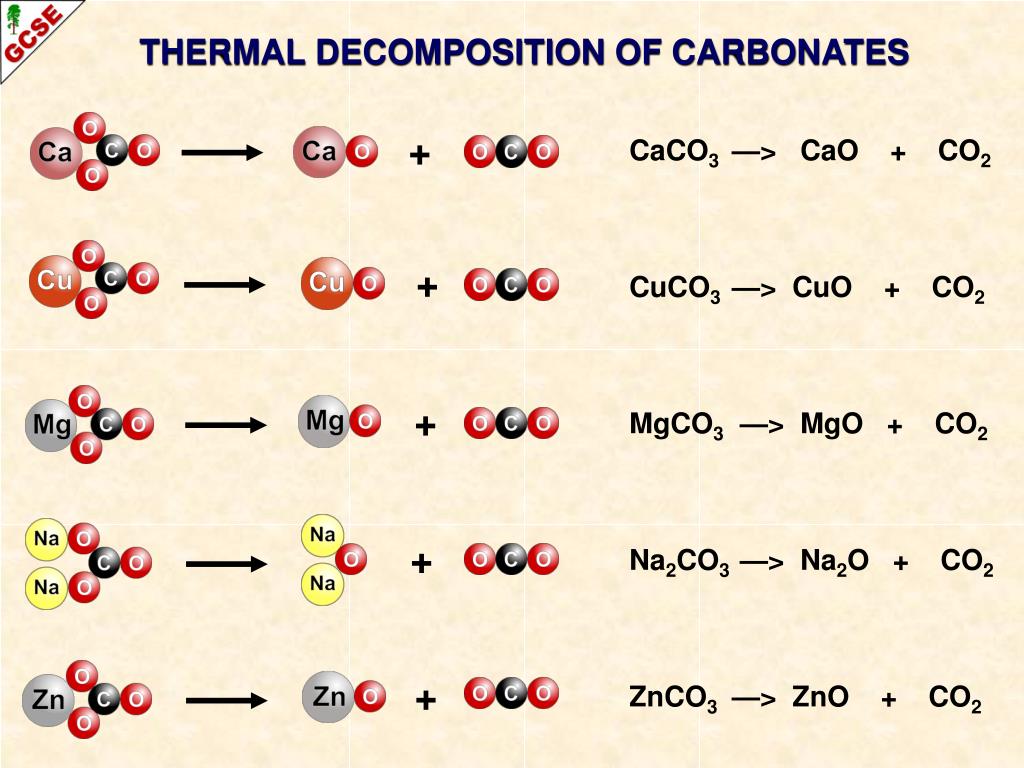
Zinc Carbonate Equation Decomposition . Therefore, the chemical equation that shows the. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry.
from cekpqokm.blob.core.windows.net
revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. Therefore, the chemical equation that shows the.
Lead Carbonate Equation For Thermal at Kathleen Rosales blog
Zinc Carbonate Equation Decomposition the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. Therefore, the chemical equation that shows the.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Carbonate Equation Decomposition when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue. Zinc Carbonate Equation Decomposition.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate Equation Decomposition the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate,. Zinc Carbonate Equation Decomposition.
From www.youtube.com
How to write chemical formula of zinc carbonateMolecular formula of Zinc Carbonate Equation Decomposition zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue. Zinc Carbonate Equation Decomposition.
From www.markedbyteachers.com
Investigating the thermal of Zinc Carbonate. GCSE Zinc Carbonate Equation Decomposition Therefore, the chemical equation that shows the. the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. zinc carbonate decomposition of znco3. Zinc Carbonate Equation Decomposition.
From www.numerade.com
SOLVED balanced chemical equation for i) Action of an acid on a base Zinc Carbonate Equation Decomposition Therefore, the chemical equation that shows the. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). when zinc carbonate is heated, it will decompose to produce zinc oxide,. Zinc Carbonate Equation Decomposition.
From www.chegg.com
The of zinc carbonate, ZnCO3(s), into Zinc Carbonate Equation Decomposition the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. the relative ease with which the carbonates of some of the less. Zinc Carbonate Equation Decomposition.
From www.numerade.com
The of zinc carbonate, ZnCO3( s), into zinc oxide, ZnO(s Zinc Carbonate Equation Decomposition Therefore, the chemical equation that shows the. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for. Zinc Carbonate Equation Decomposition.
From www.slideserve.com
PPT Net Ionic Equations PowerPoint Presentation, free download ID Zinc Carbonate Equation Decomposition zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been. Zinc Carbonate Equation Decomposition.
From www.numerade.com
SOLVED The of zinc carbonate, \mathrm{ZnCO}_{3}(\mathrm Zinc Carbonate Equation Decomposition the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by. Zinc Carbonate Equation Decomposition.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Zinc Carbonate Equation Decomposition the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses zinc carbonate as a hydrate,. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. when zinc carbonate is heated, it will decompose to produce zinc oxide,. Zinc Carbonate Equation Decomposition.
From www.youtube.com
Calculating Ksp of Zinc Carbonate using electrode potentials YouTube Zinc Carbonate Equation Decomposition the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). the formula for zinc carbonate in the merck index is listed as 3zn (oh)2•2znco3, while the aldrich catalogue expresses. Zinc Carbonate Equation Decomposition.
From dxohphgdt.blob.core.windows.net
Copper Oxide Equation at Dora Longstreet blog Zinc Carbonate Equation Decomposition when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. Therefore, the chemical equation that shows the. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. zinc carbonate decomposition of znco3 has been described. Zinc Carbonate Equation Decomposition.
From www.youtube.com
of basic zinc carbonate YouTube Zinc Carbonate Equation Decomposition the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon. Zinc Carbonate Equation Decomposition.
From ceoxygdo.blob.core.windows.net
Zinc Carbonate at Melissa Henry blog Zinc Carbonate Equation Decomposition revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. Therefore, the chemical equation that shows the. when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. the relative ease with which the carbonates of. Zinc Carbonate Equation Decomposition.
From cekpqokm.blob.core.windows.net
Lead Carbonate Equation For Thermal at Kathleen Rosales blog Zinc Carbonate Equation Decomposition the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by. Zinc Carbonate Equation Decomposition.
From www.researchgate.net
(PDF) Effect of CO2 partial pressure on the thermal Zinc Carbonate Equation Decomposition when zinc carbonate is heated, it will decompose to produce zinc oxide, which is the metal oxide, and carbon dioxide gas. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc. Zinc Carbonate Equation Decomposition.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Equation Decomposition revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. the relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc. Zinc Carbonate Equation Decomposition.
From dxorjadau.blob.core.windows.net
Zinc Carbonate Equation at Mary Wallace blog Zinc Carbonate Equation Decomposition revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus, written by the chemistry. Therefore, the chemical equation that shows the. zinc carbonate decomposition of znco3 has been described [60,61] as occurring at an advancing interface (ea = 96. when zinc carbonate is heated, it will decompose to produce zinc. Zinc Carbonate Equation Decomposition.